It's the subject that just won't go away. Which medicines should Europe's health systems pay for – and how much should they pay?
No apologies for returning to the issue, because Europe's authorities have seized on it once again, putting drug pricing at the top of the bill at a meeting of Europe's health ministers in mid-April. Pharmaceutical executives never tire of the discussion either — because they know that if it goes the wrong way, they could be out of a job, and that in the current tough economic climate, nothing can be ruled out.
Even Sanofi's CEO Olivier Brandicourt, the strong man of France's drug industry, was ready to admit — on his home turf, at a meeting in Lyon, on the eve of the health ministers' meeting — that he was "not optimistic" that the industry was getting its message across. [...]
So there is fertile ground for the health ministers' discussions of pricing — clothed in the modest figleaf of "Innovations for the benefit of the patient", as a concession to the traditional member-state insistence on keeping these decisions at national level. And Dutch health minister Edith Schippers scattered plenty of seed in advance of the meeting, with a hard-hitting paper outlining the need for action.
"The current pharmaceutical system is out-of-balance", it says, and "It is time to set a new course." Explicitly and with no apology for its radical break with tradition, it says baldly: "We should take measures to better control the price of medicines."
The paper highlights the problems that governments face. "Member states deal on an individual basis with global pharmaceutical companies, in a context of great information asymmetry between governments and industry," it points out. "Companies benefit from fragmented procurement and budgeting of medicines in Europe," and patients, people who are insured, and taxpayers throughout the European Union "are the victims." So one of the main aims should be for cooperation that can increase information-sharing and transparency about products, markets and prices, and can make better use of joint health technology assessment for reimbursement decisions is also fruitful. "If member states work together more closely," says Schippers' paper, "the imbalance at the negotiation tables can be reduced."
The industry is the culprit, despite its often helpful innovations, the paper makes clear. "The profit-oriented industry does not make drugs pricing very transparent," and "this makes a sensible discussion regarding socially acceptable drugs prices much more difficult." Drug prices no longer take account of anything else, it suggests. "The relationship between innovation and a reasonable, socially acceptable price is absent." In addition, the industry's enthusiasm for maximizing profits leads it to make "undesirable use or even misuse" of protection mechanisms linked to intellectual property.
The result, the paper alleges, is that essential medicines - including orphan medicines and oncolytics - are not affordable or not even marketed in some countries, demonstrating how "commercial interests prevail over public interest." This, says the paper, can be considered as market failure. And it provides the justification for something akin to a declaration of war: "We should therefore aim at achieving a stronger negotiating position for the purchaser in order to compel that the price of a product better reflects its actual development cost and added value."
The mechanisms envisaged are not so clearly spelled out. But the paper before ministers speaks of European cooperation as "an important condition for a system of sustainable provision of medicines" and a way of taking a stance "to bring about the necessary changes in the market authorization framework, in regulations governing supplementary market protection in addition to patent law, and reimbursement."
This isn't going to be a straightforward confrontation between governments and industry, either. The background to the debate is the growing chorus of complaints and criticisms from patients and influential civil society organisations, to which governments — ever alert to the grass-roots, and ever sensitive to the need to placate the voluble — have been paying increasing heed. [...] Read further at the orginal article HERE


 Your new post is loading...
Your new post is loading...

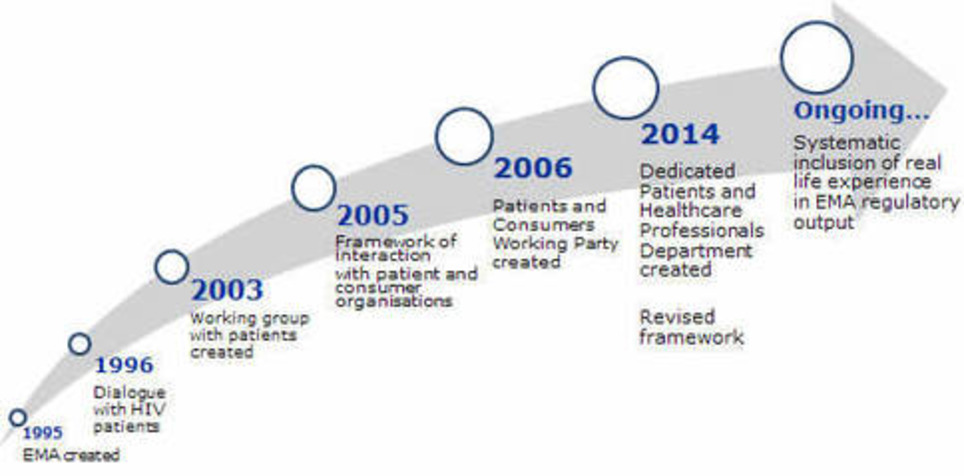





The European Medicines Agency has adopted a new framework for interaction between EMA and patients and consumers and their organizations in June of this year.
They have now published their annual report of 2015
See download of the report here
http://www.ema.europa.eu/docs/en_GB/document_library/Annual_report/2016/06/WC500209168.pdf